Zhonghua Xue Ye Xue Za Zhi. 2022 May; 43(5): 400–407.
Chinese. doi: 10.3760/cma.j.issn.0253-2727.2022.05.009
Abstract
目的
比較不同擴增體系NK細胞生物學特性的差異以及治療異基因造血幹細胞移植後(allo-HSCT)白血病復發的療效。
方法
分別採用CD3/CD52單抗擴增法和飼養層細胞擴增法誘導供者來源NK細胞大量擴增,檢測擴增前後NK細胞表型、因數分泌、細胞毒的變化規律;選取16例allo-HSCT後復發白血病患者,8例輸注CD3/CD52單抗擴增NK細胞,8例輸注飼養層細胞擴增NK細胞,觀察患者的治療反應和長期生存情況。
結果
①與CD3/CD52單抗擴增體系相比,飼養層細胞擴增體系NK細胞純度較高、NK細胞表面活化性受體DNAM-1及NKp30表達較高、抑制性受體CTLA-4表達較低,而兩種NK細胞NKG2D/CD25/CD69/Trail/PD-1/TIM-3/TIGIT表達差異無統計學意義。②兩種擴增體系NK細胞Ki-67指數均明顯增加,以飼養層細胞擴增NK細胞尤為明顯;飼養層細胞擴增NK細胞穿孔素及顆粒酶B表達水平均明顯高於CD3/CD52單抗擴增NK細胞。③16例allo-HSCT後復發白血病患者NK細胞輸注過程中均未觀察到明顯不良反應。NK細胞輸注後中位隨訪時間為2554(917~2583)d,CD3/CD52單抗擴增組中3例患者無白血病存活,5例死亡;飼養層細胞擴增組中6例患者長期存活,2例死亡。5例患者在NK細胞輸注前存在移植物抗宿主病,NK細胞輸注後移植物抗宿主病未加重甚至緩解。
結論
CD3/CD52單抗擴增與飼養層細胞擴增體系NK細胞的生物學特性具有明顯差異;NK細胞輸注對allo-HSCT後復發白血病患者的療效仍需要進一步驗證。
Keywords: 白血病, 復發, 擴增, NK細胞
異基因造血幹細胞移植(allo-HSCT)是白血病的有效治療手段,移植後復發是影響移植療效的主要原因。目前,移植後白血病復發的常規治療方法包括化療及供者淋巴細胞輸注(DLI),但DLI可使移植物抗宿主病(GVHD)的發生風險增加[1]。因此,探討新的復發後治療方法是提高白血病患者移植療效的關鍵。
自然殺傷(NK)細胞是構成機體重要的天然免疫屏障之一,無需致敏即可殺傷腫瘤細胞或感染的細胞,通過分泌穿孔素、顆粒酶等介導靶細胞殺傷[2]–[3],另一方面可通過分泌IFN-γ、TNF-α等活化適應性免疫系統而發揮協同免疫防禦作用[4]。除此之外,抗體依賴的細胞介導細胞毒性作用(ADCC)也是NK細胞效應功能發揮的重要途徑。
NK細胞效應功能強度受NK細胞活化性受體與抑制性受體的平衡、NK細胞的成熟度及對細胞因數的回應體內外多種因素調控。內源性NK細胞往往受疾病及自身HLA分子抑制等多方面影響,數量及效應功能受損,但研究證明來自健康供者的NK細胞具有殺傷腫瘤細胞功能[5]。然而,若輸注的NK細胞數量少、療程短,患者仍面臨腫瘤復發的風險。目前,CD3/CD52單抗法和K562飼養層細胞法是臨床應用較多的兩種NK細胞體外擴增方法[6]–[7]。本研究比較了以上兩種擴增體系NK細胞的生物學特性,並回顧性分析比較了兩種擴增體系NK細胞過繼性輸注治療allo-HSCT後白血病復發患者的安全性及初步療效。
物件與方法
一、NK細胞擴增方法
採集健康供者外周血,提取單個核細胞,CD3/CD52單抗擴增法參照文獻[6],飼養層細胞擴增法參照文獻[8]。
二、NK細胞採集時間點
NK細胞擴增前采外周血檢測表型及功能,體外擴增2周後檢測擴增後NK細胞表型及功能各項指標。
三、NK細胞表型及功能評估方法
- 表型評估:外周血來源的單個核細胞及擴增後的NK細胞,加入膜表面抗體標記,避光孵育30 min,溶血並PBS洗滌後上機檢測。抗體選擇見表1。
表1
流式細胞術評估NK細胞表型及功能所用抗體
| 生產商 | 抗體 | 標誌抗體 | 克隆號 |
| BD | CD56 | BUV737 | NCAM16.2 |
| BD | CD158a | BV421 | HP-3E4 |
| BD | CD45 | V500 | HI30 |
| Biolegend | NKG2D | BV605 | 1D11 |
| Biolegend | CXCR3 | BV650 | G025H7 |
| Biolegend | CTLA-4 | BV785 | BNL3 |
| BD | CD158e | FITC | DX9 |
| Biolegend | CD158f | PE | UP-R1 |
| Biolegend | CD57 | PE-CF594 | 12G5 |
| BD | PD-1 | PE-Cy7 | EH12.1 |
| MACS | NKG2C | AF647 | FAB138N |
| Biolegend | CD3 | AF700 | UCHT1 |
| BD | CD16 | APC-Cy7 | 3G8 |
| BD | CD25 | BUV395 | 2A3 |
| BD | CD69 | BUV737 | FN50 |
| Biolegend | 4-1BB | BV421 | 4B4-1 |
| Biolegend | CXCR4 | BV605 | 12G5 |
| Biolegend | CD27 | BV650 | O323 |
| BD | TIM-3 | BV711 | 7D3 |
| Biolegend | LFA-1 | FITC/AF488 | m24 |
| BD | CD94 | Percp-Cy5.5 | HP-3D9 |
| BD | NKP46 | PE | 9E2/NKp46 |
| Biolegend | CD96 | PE-CF594 | NK92.39 |
| BD | CD62L | PE-Cy7 | DREG-56 |
| BD | DNAM-1 | APC/AF647 | DX11 |
| Biolegend | CD56 | APC-Cy7 | HCD56 |
| BD | NKP30 | BV421 | p30-15 |
| Biolegend | TIGIT | BV605 | A15153G |
| Biolegend | CX3CR1 | BV650 | 2A9-1 |
| BD | CD107a | BV786 | H4A3 |
| RD | TRAIL | FITC | FAB687G |
| Biolegend | NKP44 | Percp-Cy5.5 | P44-8 |
| MACS | NKG2A | PE-Cy7 | REA110 |
| Biolegend | NKp80 | APC/AF647 | 5D12 |
- 功能評估:對於NK細胞擴增前後細胞增殖及細胞毒的評估,則在膜表面抗體標記後,按照Cytofix/Cytoperm試劑盒(美國Becton Dickinson公司產品)說明固定破膜後標記細胞內免疫因數。抗體選擇見表1。
四、入組病例及移植方案
- 病例:16例患者納入本研究。8例患者輸注CD3/CD52擴增NK細胞,均為2011年2月至2014年2月在北京大學血液病研究所進行allo-HSCT且移植後白血病復發患者;8例患者輸注K562飼養層擴增NK細胞,為2016年8月至2018年6月在北京大學血液病研究所進行allo-HSCT且移植後白血病復發患者。所有患者均簽署知情同意書。
- 預處理方案:全部採用常規的改良BU/CY(白消安/環磷醯胺)方案,HLA配型不合患者移植預處理方案中加用抗胸腺細胞球蛋白(ATG)[9]。
- 幹細胞的動員和採集:全部患者採用骨髓聯合外周血幹細胞移植,所有患者均以G-CSF動員5~6 d(−3 d開始),劑量為5 µg/kg[10]–[11]。
- 急性GVHD的預防:採用環孢素A(CsA)聯合黴酚酸酯(MMF)及短程甲氨蝶呤(MTX)方案。
- 植活標準:連續3 d中性粒細胞絕對計數(ANC)≥0.5×109/L為粒細胞植活,PLT≥20×109/L連續7 d且脫離血小板輸注為血小板植活[12]。
五、患者移植後微小殘留病(MRD)監測
多參數流式細胞術(MFC)和即時螢光定量PCR(RT-PCR)用來檢測MRD[13]。MRD陽性:①MFC連續兩次陽性或者WT1連續兩次陽性抑或是在同一標本中MFC或WT1陽性各1次;②移植後出現基因重排或突變。MRD緩解:在NK細胞輸注後達到MRD陰性狀態並維持至少1個月[14]。對於骨髓中原始細胞>5%定義為血液學復發。
六、隨訪
通過查閱住院/門診病歷及電話隨訪方式獲取患者生存資料。
七、統計學處理
採用GraphPad Prism8分析資料,採用非配對t檢驗進行單因素分析,P<0.05為差異有統計學意義。
結果
一、兩種擴增體系NK細胞生物學差異
- 兩種擴增體系NK細胞活化性受體的表達:CD3/CD52單抗擴增組NK細胞在CD45陽性淋巴亞群中的中位占比為18.9%,而飼養層細胞擴增的NK細胞中位數比例為85.0%,純度更高。通過對不同體系擴增前後NK細胞活化性受體的表達評估,我們發現滋養層擴增的NK細胞的活化性受體DNAM-1及NKp30的表達都明顯高於CD3/CD52單抗擴增組(P=0.016,P=0.006),而NKG2D、Trail占NK細胞的百分比在兩組間差異無統計學意義(P>0.05)(圖1、圖2)。CD25及CD69分別被認為是NK細胞早期活化和晚期活化的重要指標[15]。在我們的佇列中,CD25及CD69占NK細胞百分比在CD3/CD52單抗擴增組及飼養層擴增組均無明顯差別(P>0.05),提示兩種擴增NK細胞儘管在活化性受體表達方面存在差異,但兩者綜合的活化程度相當(圖1、圖2)。
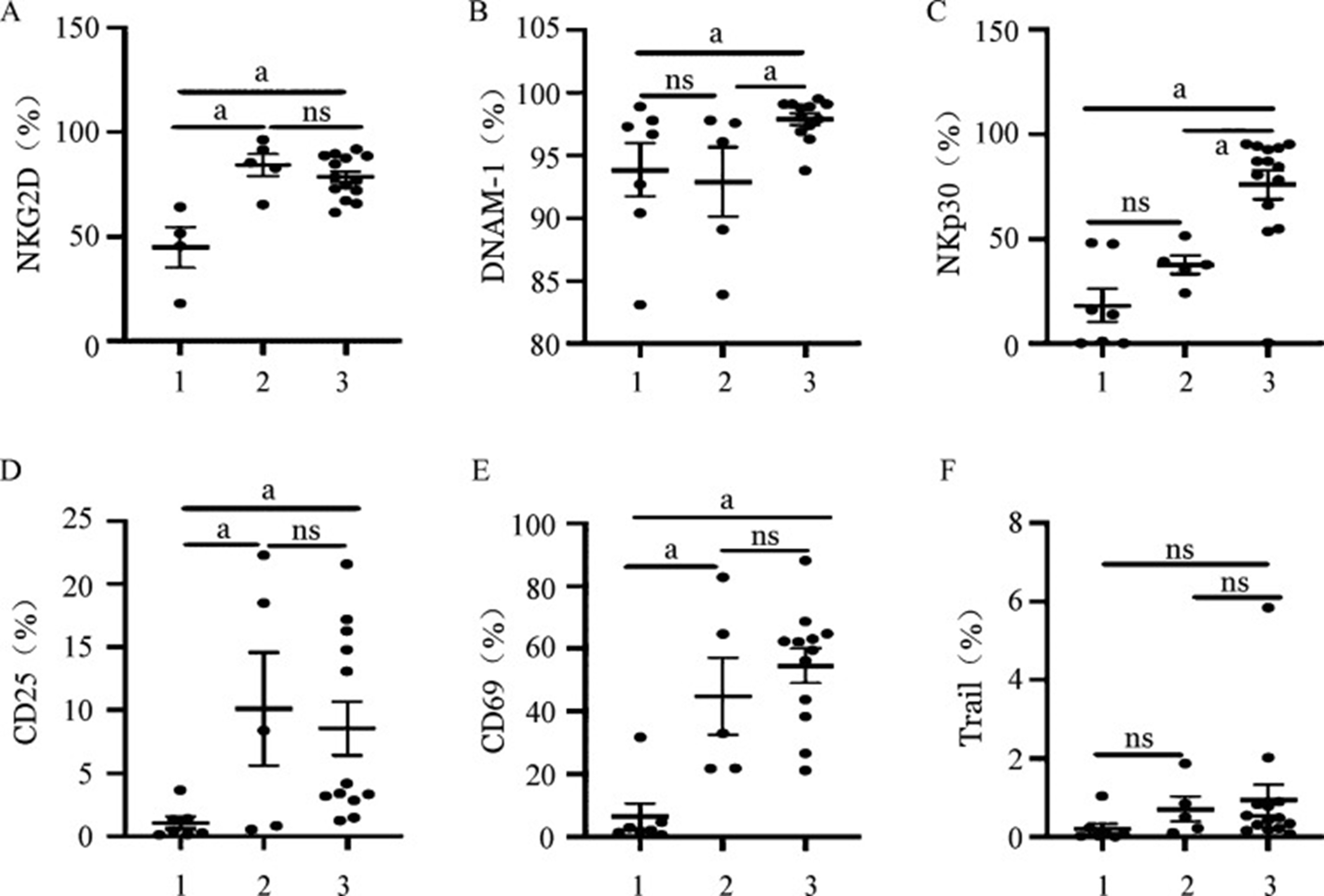 圖1兩種不同擴增體系NK細胞活化性受體表達
圖1兩種不同擴增體系NK細胞活化性受體表達
1、2、3分別為擴增前組、CD3/CD52單抗擴增組、飼養層細胞擴增組。ns:差異無統計學意義;a P<0.05
兩種不同擴增體系NK細胞活化性受體平均螢光強度(MFI)
1、2、3分別為擴增前組、CD3/CD52單抗擴增組、飼養層細胞擴增組。ns:差異無統計學意義;a P<0.05
- 兩種擴增體系NK細胞抑制性受體的表達:兩種體系擴增的NK細胞抑制性受體CTLA4、PD-1、TIM-3的平均螢光強度(MFI)較擴增前均明顯上調(P<0.001,P=0.003,P=0.001),然而Tigit的平均螢光強度無明顯變化(P>0.05)(圖3)。飼養層細胞擴增組NK細胞CTLA4(占NK細胞百分比)的表達明顯低於CD3/CD52單抗擴增組(P<0.001),而其他抑制性受體(PD-1、TIM-3、Tigit)在兩組間無論是平均螢光強度還是在NK細胞中占比差異均無統計學意義(P>0.05)。提示體外擴增誘導NK細胞活化的同時可能一定程度上伴隨功能耗竭。
 圖3兩種不同擴增體系NK細胞抑制性受體平均螢光強度(MFI)
圖3兩種不同擴增體系NK細胞抑制性受體平均螢光強度(MFI)
1、2、3分別為擴增前組、CD3/CD52單抗擴增組、飼養層細胞擴增組。ns:差異無統計學意義;a P<0.05
- 兩種擴增體系NK細胞趨化型受體的表達:NK細胞向組織臟器中的遷移依賴於其表面的各種黏附性分子及趨化性受體的表達。為此,我們評估了不同擴增體系對NK細胞表面黏附性分子及趨化性受體表達的影響,結果顯示在飼養層細胞擴增組NK細胞中CXCR3、CD96的比例明顯高於CD3/CD52單抗擴增組(P=0.004,P=0.037),CX3CR1及4-1BB在NK細胞的表達比例在飼養層擴增組更低,CXCR4、CD94、CD27及CD62L的比例在兩種擴增體系NK細胞間差異均無統計學意義(P>0.05),提示從黏附分子和趨化受體表達角度,兩種擴增方法各有利弊(圖4)。
 圖4兩種不同擴增體系NK細胞趨化性受體表達
圖4兩種不同擴增體系NK細胞趨化性受體表達
1、2、3分別為擴增前組、CD3/CD52單抗擴增組、飼養層細胞擴增組。MFI:平均螢光強度;ns:差異無統計學意義;a P<0.05
- 兩種擴增體系NK細胞功能表達差異:我們通過檢測擴增NK細胞的Ki-67指標評估其不同體系的擴增能力。結果顯示,飼養層擴增NK細胞的增殖潛能明顯高於CD3/CD52單抗擴增NK細胞(P=0.033),穿孔素及顆粒酶B的表達水準也明顯高於抗體擴增NK細胞(圖5)。
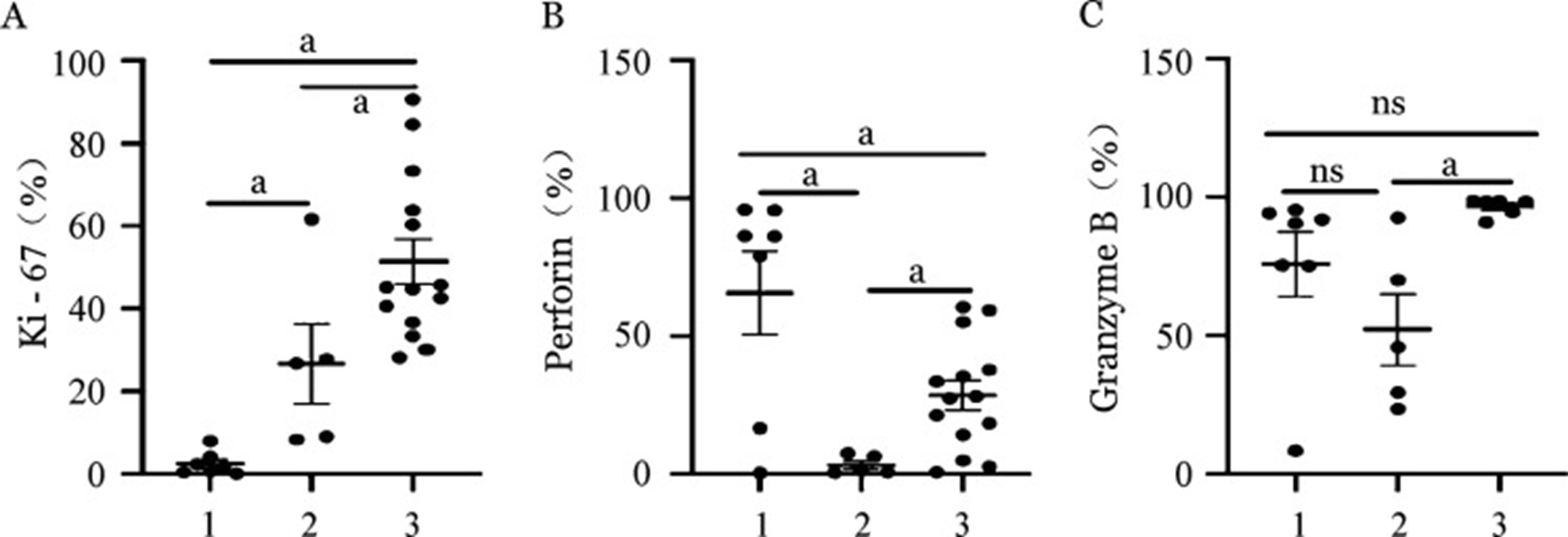 圖5兩種不同擴增體系NK細胞生物學功能比較
圖5兩種不同擴增體系NK細胞生物學功能比較
1、2、3分別為擴增前組、CD3/CD52單抗擴增組、飼養層細胞擴增組。MFI:平均螢光強度;ns:差異無統計學意義;a P<0.05。Perforin:穿孔素;Granzyme B:顆粒酶B
二、入組患者的臨床特點
CD3/CD52單抗擴增組中男5例、女3例,4例接受同胞HLA全相合allo-HSCT,4例接受haplo-HSCT,7例患者移植時處於完全緩解狀態,1例移植前為復發狀態。至2021年6月隨訪終止時,中位隨訪時間為2 554 d,共有3例患者死亡。
飼養層擴增組中男4例、女4例,5例接受同胞HLA全相合allo-HSCT,3例接受haplo-HSCT,8例患者移植時均處於完全緩解狀態。患者首次接受供者來源NK細胞輸注的中位時間是移植後482 d。至2021年6月隨訪終止時,中位隨訪時間1 015.5(917~1 068)d,共有2例患者死亡。
患者主要臨床特點和NK細胞治療前情況見表2。
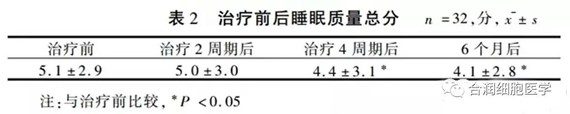
表2
16例異基因造血幹細胞移植後復發白血病患者的一般資料及NK細胞治療前情況
例號 性別 年齡 疾病類型 移植模式 急性GVHD NK細胞輸注前化療方案 NK細胞輸注前疾病狀態 1 男 37 AML-M2 HLA全相合 無 FLAG,HAA 血液學復發 2 男 52 B-ALL 單倍型 無 COPD 血液學復發 3 男 26 AML-M2 HLA全相合 無 HAA 髓外復發 4 女 12 AML-M2 單倍型 Ⅰ度,皮膚Ⅱ度 無 MRD(+) 5 男 21 MDS-RAEB2 單倍型 Ⅳ度(皮膚、肝) 無 MRD(+) 6 女 46 AML-M2 單倍型 無 無 MRD(+) 7 男 47 B-ALL HLA全相合 無 COPD 髓外復發,MRD(+) 8 女 33 AML-M2 HLA全相合 腸道Ⅰ度 無 血液學復發 9 女 40 AML-M2 HLA全相合 無 無 MRD(+) 10 男 31 AML-M2 單倍型 Ⅰ度,皮膚Ⅱ度 AA MRD(+) 11 女 54 AML-M4 HLA全相合 無 無 血液學復發,髓外復發,MRD(+) 12 女 24 AML-M5 單倍型 皮膚Ⅰ度 AA MRD(+) 13 男 31 AML-M2 單倍型 無 無 血液學復發 14 男 30 AML-M2 HLA全相合 無 FLAG MRD(+) 15 男 49 AML-M2 HLA全相合 無 無 血液學復發 16 女 33 AML-M2 HLA全相合 無 無 MRD(+) 注:AML:急性髓系白血病;B-ALL:急性B淋巴細胞白血病;MDS-RAEB2:骨髓增生異常綜合征-難治性貧血伴有原始細胞增多2型;MRD:微小殘留病;FLAG方案:氟達拉賓+阿糖胞苷+G-CSF;HAA方案:高三尖杉酯堿+阿糖胞苷+阿柔比星;COPD方案:環磷醯胺+長春新堿+柔紅黴素+潑尼松;AA方案:阿柔比星+阿糖胞苷;GVHD:移植物抗宿主病
三、安全性評價
兩組患者在NK細胞輸注後均未觀察到發熱、血象下降、臟器功能損害等不良反應。
四、NK細胞治療情況及療效評價
飼養層細胞擴增組平均接受NK細胞輸注次數為1.25次,CD3/CD52單抗擴增組為2.4次。兩組共觀察到血液學復發5例(伴髓外復發1例),單純MRD持續陽性8例,單純髓外復發1例,髓外復發合併MRD陽性1例。患者NK細胞中位輸注次數為1次,移植後接受NK細胞輸注治療的中位時間為移植後434(103~1 225)d。NK細胞輸注後中位隨訪時間為2 554(917~2 583)d,復發患者緩解率為16.7%(1/6),MRD陽性患者轉陰率為54.5%(6/11),無白血病生存(LFS)率為43.75%(7/16),總生存(OS)率為56.25%(9/16)。對於持續緩解的患者,NK細胞治療後有6例患者接受化療聯合DLI,4例行2次移植。
飼養層擴增組8例患者中6例有治療反應,其中HLA全相合移植4例,haplo-HSCT 2例,輸注過程均無明顯不良反應,其中5例患者至今無白血病長期生存,1例患者因血液復發死亡。CD3/CD52單抗擴增組8例患者中4例有治療反應,其中HLA全相合移植2例,haplo-HSCT 2例,輸注過程均無明顯不良反應,其中3例患者至今無白血病長期生存,1例因重症肺炎導致呼吸衰竭而死亡。
而對NK細胞回輸治療無反應患者中,CD3/CD52單抗擴增組8例患者中4例無治療反應(1例為持續MRD陽性,3例為血液學復發),經化療、DLI等治療均未得到緩解,4例均死亡(死於中樞神經系統感染、重度GVHD合併TMA各1例,血液學復發2例)。在飼養層細胞擴增組8例患者中有2例無治療反應(均為MRD持續陽性),經化療後分子學及血液學復發指標均未得到緩解,其中1例NK細胞輸注後MRD仍持續陽性,但後續再行DLI治療後MRD轉陰,至今無白血病生存;另一例患者死于髓外復發。
CD3/CD52單抗擴增組、飼養層擴增組NK細胞輸注後MRD轉陰率分別為50%(2/4)、50%(3/6)。
滋養層擴增組的例11在接受NK細胞治療前有重度慢性GVHD(累及肺、皮膚、口腔等器官);另有3例在移植後分別表現為皮膚、口腔及指甲、皮膚及肺部急性GVHD表現,輸注後症狀緩解。
在CD3/CD52單抗擴增組,我們觀察到3例患者GVHD症狀在NK細胞輸注後緩解,提示NK細胞可能有抗GVHD的功能。NK細胞治療情況及療效見表3。
表3
16例異基因造血幹細胞移植後復發白血病患者的NK細胞治療情況及隨訪結果
例號 NK細胞輸注次數 NK細胞輸注量(×109/L) 療效評估 隨訪時間(d) 隨訪結果 1 2 6.20 無效 2 560 死亡(中樞神經系統感染) 2 1 4.76 無效 2 560 死亡(重度GVHD/TMA) 3 5 6.62 有效 2 583 存活 4 1 4.19 有效 2 540 存活 5 3 6.02 有效 2 540 存活 6 1 0.36 無效 2 554 死亡(血液學復發) 7 5 10.31 有效 2 554 死亡(重症肺炎) 8 1 6.44 無效 2 552 死亡(血液學復發) 9 1 6.18 有效 1 068 存活 10 2 7.02 有效 1 064 存活 11 1 7.10 有效 1 019 存活 12 1 4.40 無效 917 存活 13 1 7.32 有效 1 012 死亡(血液學復發) 14 2 3.14 無效 994 死亡(血液學復發) 15 1 3.82 有效 917 存活 16 1 2.48 有效 1 047 存活 注:GVHD:移植物抗宿主病;TMA:血栓性微血管病
討論
NK細胞是機體天然免疫屏障的重要組成,是抗病毒和抗腫瘤的主力軍之一[7]。除此之外,供者KIR不相合的NK細胞輸注至受者體內,可通過抑制受者來源的抗原提呈細胞及T細胞,降低移植物抗宿主病的發生。因此,過繼性輸注NK細胞的免疫療法已成為了潛在抗腫瘤的有效治療方式。然而,由於外周血NK細胞比例低,獲取數量少,使得NK細胞治療療效受限。體外細胞擴增體系的發展使得NK細胞可在短期(2~3周)內獲取,並且輸注的NK細胞在動物及人體中均顯示發揮抗腫瘤效應的同時不增加GVHD的發生率[5]。但是,不同擴增體系產出的NK細胞生物學特性和臨床療效存在較大差異[16]–[17]。Masuyama等[6]首次通過CD3/CD52單抗體外刺激外周血單個核細胞後聯合自體血漿及IL-2,在無飼養層細胞體系中實現了NK細胞的量級擴增,且抗腫瘤功能增強。儘管該無飼養層體系擴增NK細胞比例相對較高,但培養14 d後的擴增細胞中CD8+T細胞及CD4+T細胞比例中位值分別為60%、15%。隨著體外擴增體系的完善,體外飼養層細胞擴增體系擴增NK細胞效率更佳[14]。然而體外飼養層細胞擴增體系需要大量且持續的因數刺激,當輸注至體內後,細胞因數刺激驟失將導致輸注後NK細胞代謝加快。本研究首次對CD3/CD52單抗擴增NK細胞與K562飼養層擴增NK細胞的生物學差異進行探討,結果顯示,以K562飼養層擴增的NK細胞純度較高、T細胞含量較低。兩種不同擴增體系NK細胞活化性受體表達及KI-67均明顯高於擴增前NK細胞,說明NK細胞擴增同時伴隨著功能活化,尤以飼養層擴增組NK細胞的增殖水準及活化性受體DNAM-1及NKP30的表達水準更高,而抑制性受體CTLA4表達明顯低於CD3/CD52單抗擴增組,抑制性受體PD-1、Tigit及Tim-3的表達在兩組間無明顯差異。以上結果提示飼養層擴增的NK細胞的抗腫瘤能力可能更強。值得注意的是,兩組擴增後NK細胞的活化性受體明顯增加,抑制性受體相較於擴增前組也明顯上調,這與Judge等[18]的報導一致,可能是擴增NK細胞為了防止過度活化的適應性調控。
既往有研究前瞻性評估不同體系擴增的NK細胞在治療AML中的療效,結果提示過繼性NK細胞輸注在治療低白血病負荷中有優勢[8],[14],然而尚未有研究報導不同體系擴增的NK細胞在治療AML中的療效差異。在本研究中,兩種不同擴增體系NK細胞在生物學特性上存在差異,兩種擴增體系NK細胞輸注對移植後血液學復發白血病患者均無顯著療效;而對持續MRD陽性患者來說,在CD3/CD52單抗擴增組和飼養層擴增組,分別有50%(2/4)、50%(3/6)的MRD陽性患者NK細胞治療後轉陰,提示NK細胞治療可能有清除體內MRD作用,並且以飼養層擴增NK細胞效果更好,這可能與飼養層擴增的NK細胞活化性受體表達更高有關;與此同時,本研究結果表明飼養層擴增NK細胞趨化型因數受體CXCR3與CXCR4的表達均明顯高於CD3/CD52單抗擴增NK細胞,提示飼養層擴增NK細胞的遷移速度與向骨髓的趨化能力更高,也更有利於擴增NK細胞向腫瘤部位的遷移[19]。同時,Denman等[20]的研究表明裝載IL-21的K562細胞作為飼養層細胞體外擴增NK細胞的端粒酶更長,提示滋養層擴增NK細胞可能體內存活時間更長。
在本研究中,兩種擴增體系NK細胞治療移植後復發白血病患者並未引起或加重GVHD,且兩組均有部分患者的急慢性GVHD症狀在NK細胞輸注後得以緩解,提示NK細胞輸注治療的安全性良好。既往研究表明NK細胞在抗白血病細胞的同時具有抗GVHD效應[5],這得益於NK細胞對效應性T細胞的功能和數量制約:一方面NK細胞可殺傷供者來源的T細胞,同時可通過殺傷不成熟的樹突狀細胞減少抗原提呈[21]–[22]。與此同時,異基因來源的NK細胞不損傷患者的靶器官,因此不會發生GVHD和細胞因數風暴(CRS)。
本研究結果初步顯示,CD3/CD52單抗擴增與飼養層細胞擴增體系NK細胞的表型和功能具有明顯差異,兩種擴增體系NK細胞在治療移植後持續MRD陽性均有效。本研究為回顧性研究且佇列樣本數少,因此NK細胞輸注對allo-HSCT後復發白血病患者的療效仍需要進一步驗證。
Footnotes
利益衝突 本研究所用NK細胞產品由北京賽傲生物技術有限公司提供
作者貢獻聲明 曹勳紅:資料收集、資料分析、文章撰寫;趙翔宇:研究設計、寫作指導;其他:資料收集
References
- Frey NV, Porter DL. Graft-versus-host disease after donor leukocyte infusions: presentation and management[J] Best Pract Res Clin Haematol. 2008;21(2):205–222. doi: 10.1016/j.beha.2008.02.007. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Krzewski K, Gil-Krzewska A, Nguyen V, et al. Lamp1/cd107a is required for efficient perforin delivery to lytic granules and nk-cell cytotoxicity[J] Blood. 2013;121(23):4672–4683. doi: 10.1182/blood-2012-08-453738. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Fehniger TA, Cai SF, Cao XF, et al. Acquisition of murine nk cell cytotoxicity requires the translation of a pre-existing pool of granzyme b and perforin mrnas[J] Immunity. 2007;26(6):798–811. doi: 10.1016/j.immuni.2007.04.010. [PubMed] [CrossRef] [Google Scholar]
- Whitmire JK, Tan JT, Whitton JL. Interferon-γ acts directly on CD8+T cells to increase their abundance during virus infection[J] J Exp Med. 2005;201(7):1053–1059. doi: 10.1084/jem.20041463. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants[J] Science. 2002;295(5562):2097–2100. doi: 10.1126/science.1068440. [PubMed] [CrossRef] [Google Scholar]
- Masuyama J, Murakami T, Iwamoto S, et al. Ex vivo expansion of natural killer cells from human peripheral blood mononuclear cells co-stimulated with anti-CD3 and anti-CD52 monoclonal antibodies[J] Cytotherapy. 2016;18(1):80–90. doi: 10.1016/j.jcyt.2015.09.011. [PubMed] [CrossRef] [Google Scholar]
- 曹 勳紅, 余 星星, 胡 利娟, et al. 異基因造血幹細胞移植後NK細胞免疫重建的研究進展[J] 現代免疫學2019;39(1):64–67. [PubMed] [Google Scholar]
- Vela M, Corral D, Carrasco P, et al. Haploidentical IL-15/41BBL activated and expanded natural killer cell infusion therapy after salvage chemotherapy in children with relapsed and refractory leukemia[J] Cancer Lett. 2018;422:107–117. doi: 10.1016/j.canlet.2018.02.033. [PubMed] [CrossRef] [Google Scholar]
- Lu DP, Dong L, Wu T, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation[J] Blood. 2006;107(8):3065–3073. doi: 10.1182/blood-2005-05-2146. [PubMed] [CrossRef] [Google Scholar]
- Wang Y, Chang YJ, Xu LP, et al. Who is the best donor for a related HLA haplotype-mismatched transplant?[J] Blood. 2014;124(6):843–850. doi: 10.1182/blood-2014-03-563130. [PubMed] [CrossRef] [Google Scholar]
- Wang Y, Liu QF, Xu LP, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study[J] Blood. 2015;125(25):3956–3962. doi: 10.1182/blood-2015-02-627786. [PubMed] [CrossRef] [Google Scholar]
- Huang XJ, Liu DH, Liu KY, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies[J] Bone Marrow Transplant. 2006;38(4):291–297. doi: 10.1038/sj.bmt.1705445. [PubMed] [CrossRef] [Google Scholar]
- Wang Y, Chen H, Chen J, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China[J] Cancer Lett. 2018;438:63–75. doi: 10.1016/j.canlet.2018.08.030. [PubMed] [CrossRef] [Google Scholar]
- Zhao XY, Jiang Q, Jiang H, et al. Expanded clinical-grade membrane-bound IL-21/4-1BBL NK products exhibit activity against acute myeloid leukemia in vivo[J] Eur J Immunol. 2020;50(9):1374–1385. doi: 10.1002/eji.201948375. [PubMed] [CrossRef] [Google Scholar]
- Clausen J, Vergeiner B, Enk M, et al. Functional significance of the activation-associated receptors cd25 and cd69 on human nk-cells and nk-like t-cells[J] Immunobiology. 2003;207(2):85–93. doi: 10.1078/0171-2985-00219. [PubMed] [CrossRef] [Google Scholar]
- Yang Y, Badeti S, Tseng HC, et al. Superior expansion and cytotoxicity of human primary NK and CAR-NK cells from various sources via enriched metabolic pathways[J] Mol Ther Methods Clin Dev. 2020;18:428–445. doi: 10.1016/j.omtm.2020.06.014. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Bachanova V, Cooley S, Defor TE, et al. Clearance of acute myeloid leukemia by haploidentical natural killer cells is improved using IL-2 diphtheria toxin fusion protein[J] Blood. 2014;123(25):3855–3863. doi: 10.1182/blood-2013-10-532531. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Judge SJ, Dunai C, Aguilar EG, et al. Minimal PD-1 expression in mouse and human NK cells under diverse conditions[J] J Clin Invest. 2020;130(6):3051–3068. doi: 10.1172/JCI133353. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Kim J, Kim JS, Lee HK, et al. CXCR3-deficient natural killer cells fail to migrate to B16F10 melanoma cells[J] Int Immunopharmacol. 2018;63:66–73. doi: 10.1016/j.intimp.2018.07.026. [PubMed] [CrossRef] [Google Scholar]
- Denman CJ, Senyukov VV, Somanchi SS, et al. Membrane-bound il-21 promotes sustained ex vivo proliferation of human natural killer cells[J] PLoS One. 2012;7(1):e30264. doi: 10.1371/journal.pone.0030264. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- Terrazzano G, Pisanti S, Grimaldi S, et al. Interaction between natural killer and dendritic cells: the role of cd40, cd80 and major histocompatibility complex class i molecules in cytotoxicity induction and interferon-gamma production[J] Scand J Immunol. 2004;59(4):356–362. doi: 10.1111/j.0300-9475.2003.01387.x. [PubMed] [CrossRef] [Google Scholar]
- Ghadially H, Ohana M, Elboim M, et al. Nk cell receptor nkp46 regulates graft-versus-host disease[J] Cell Rep. 2014;7(6):1809–1814. doi: 10.1016/j.celrep.2014.05.011. [PMC free article] [PubMed] [CrossRef] [Google Scholar]
- 兩種擴增體系NK細胞功能表達差異:我們通過檢測擴增NK細胞的Ki-67指標評估其不同體系的擴增能力。結果顯示,飼養層擴增NK細胞的增殖潛能明顯高於CD3/CD52單抗擴增NK細胞(P=0.033),穿孔素及顆粒酶B的表達水準也明顯高於抗體擴增NK細胞(圖5)。
- 兩種擴增體系NK細胞趨化型受體的表達:NK細胞向組織臟器中的遷移依賴於其表面的各種黏附性分子及趨化性受體的表達。為此,我們評估了不同擴增體系對NK細胞表面黏附性分子及趨化性受體表達的影響,結果顯示在飼養層細胞擴增組NK細胞中CXCR3、CD96的比例明顯高於CD3/CD52單抗擴增組(P=0.004,P=0.037),CX3CR1及4-1BB在NK細胞的表達比例在飼養層擴增組更低,CXCR4、CD94、CD27及CD62L的比例在兩種擴增體系NK細胞間差異均無統計學意義(P>0.05),提示從黏附分子和趨化受體表達角度,兩種擴增方法各有利弊(圖4)。
- 兩種擴增體系NK細胞抑制性受體的表達:兩種體系擴增的NK細胞抑制性受體CTLA4、PD-1、TIM-3的平均螢光強度(MFI)較擴增前均明顯上調(P<0.001,P=0.003,P=0.001),然而Tigit的平均螢光強度無明顯變化(P>0.05)(圖3)。飼養層細胞擴增組NK細胞CTLA4(占NK細胞百分比)的表達明顯低於CD3/CD52單抗擴增組(P<0.001),而其他抑制性受體(PD-1、TIM-3、Tigit)在兩組間無論是平均螢光強度還是在NK細胞中占比差異均無統計學意義(P>0.05)。提示體外擴增誘導NK細胞活化的同時可能一定程度上伴隨功能耗竭。